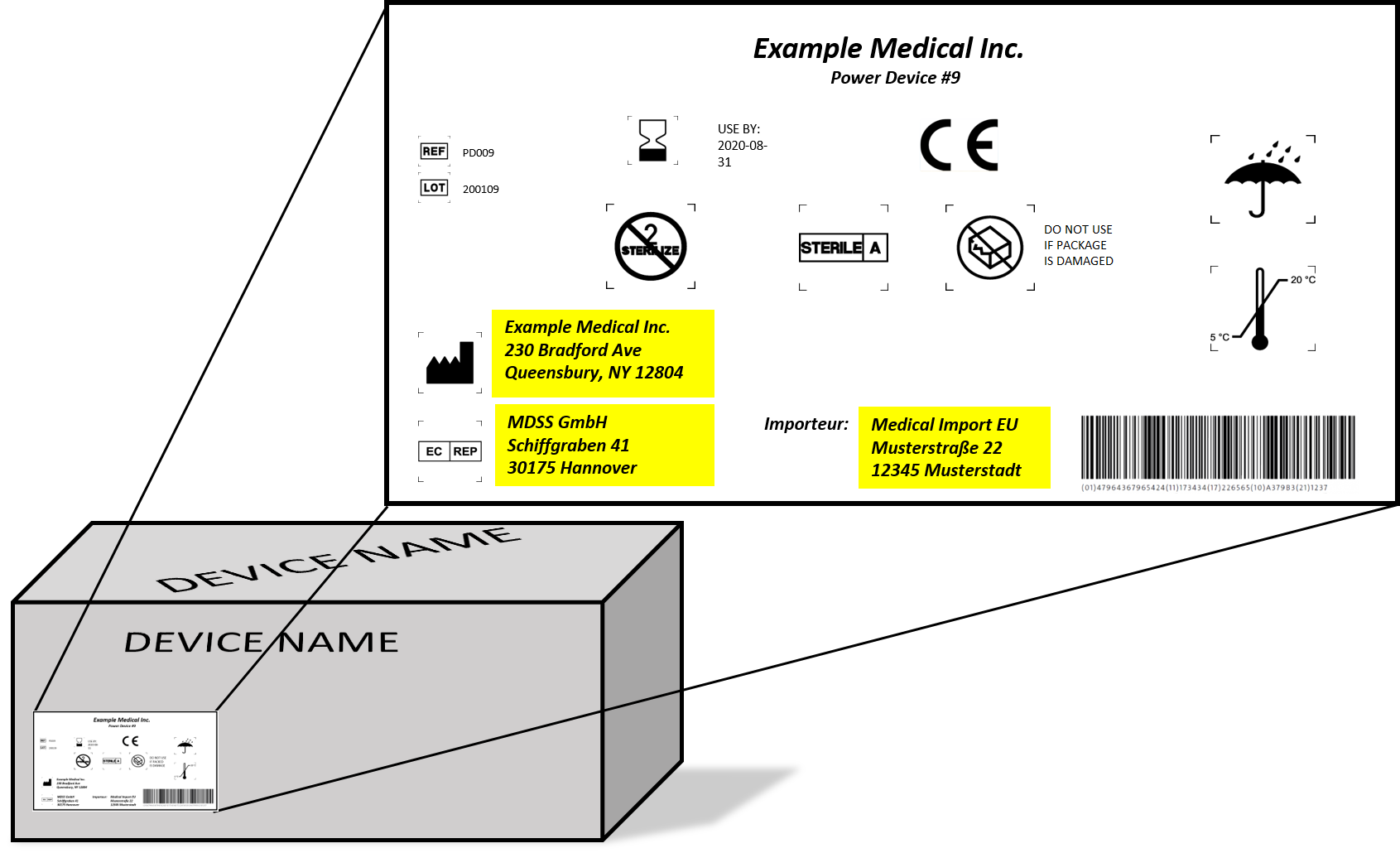
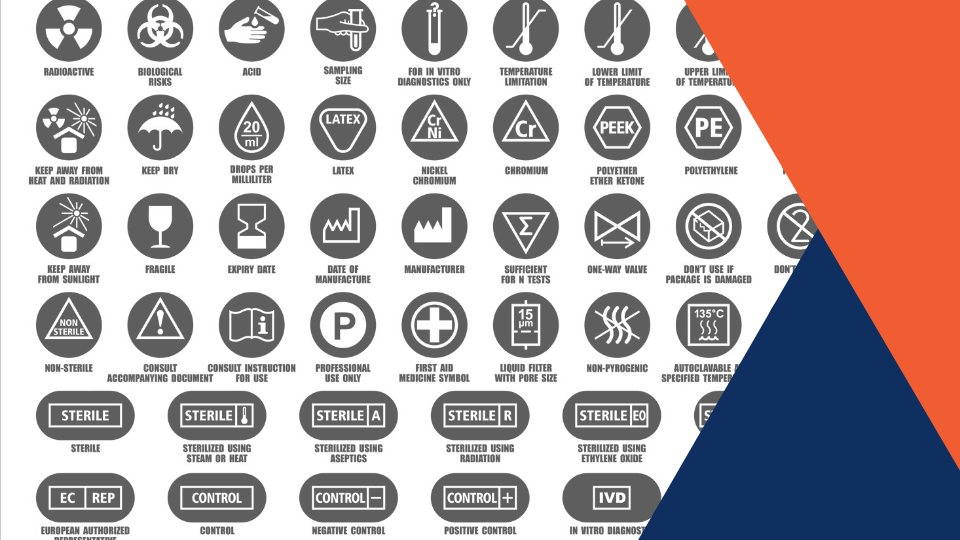
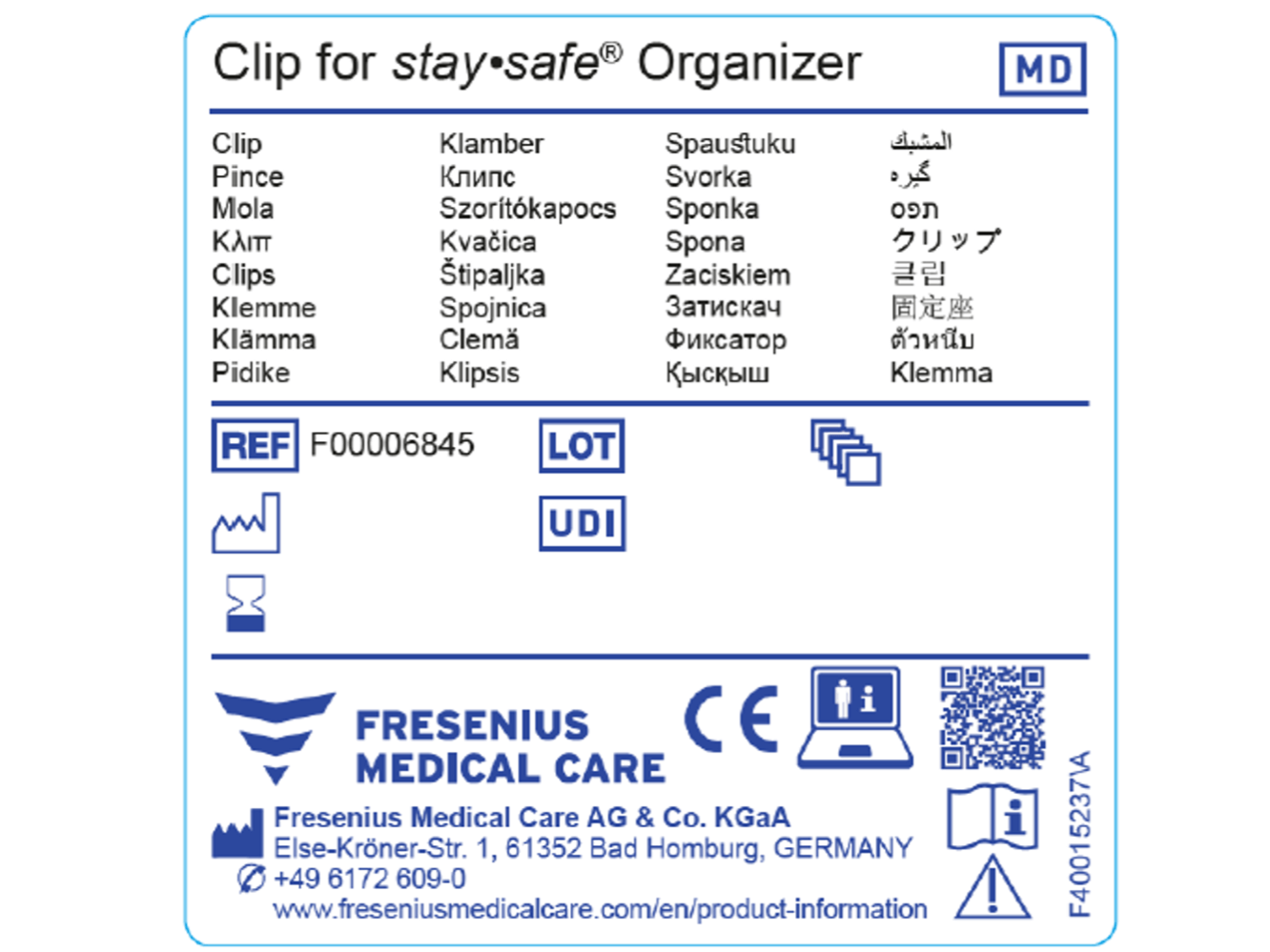
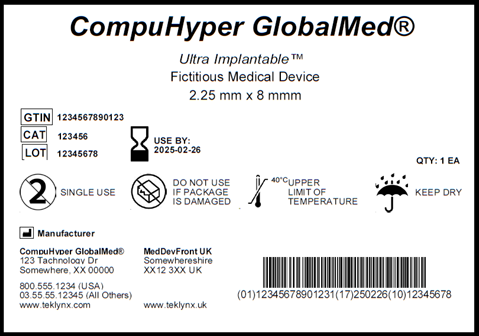
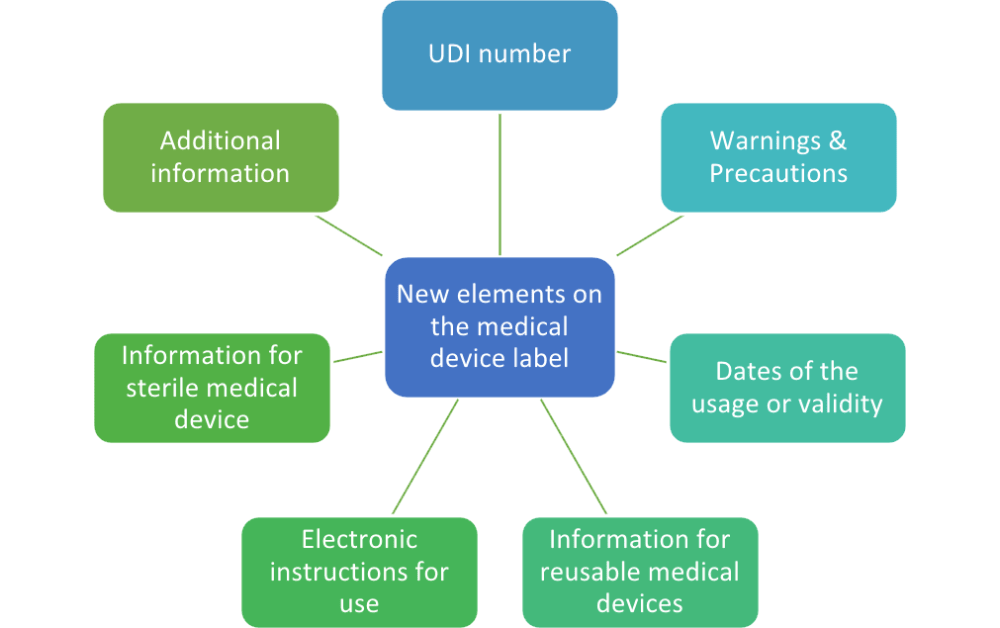
MDR Checklist Labelling & IFU Requirements · MDlaw – Information platform on European medical device regulations

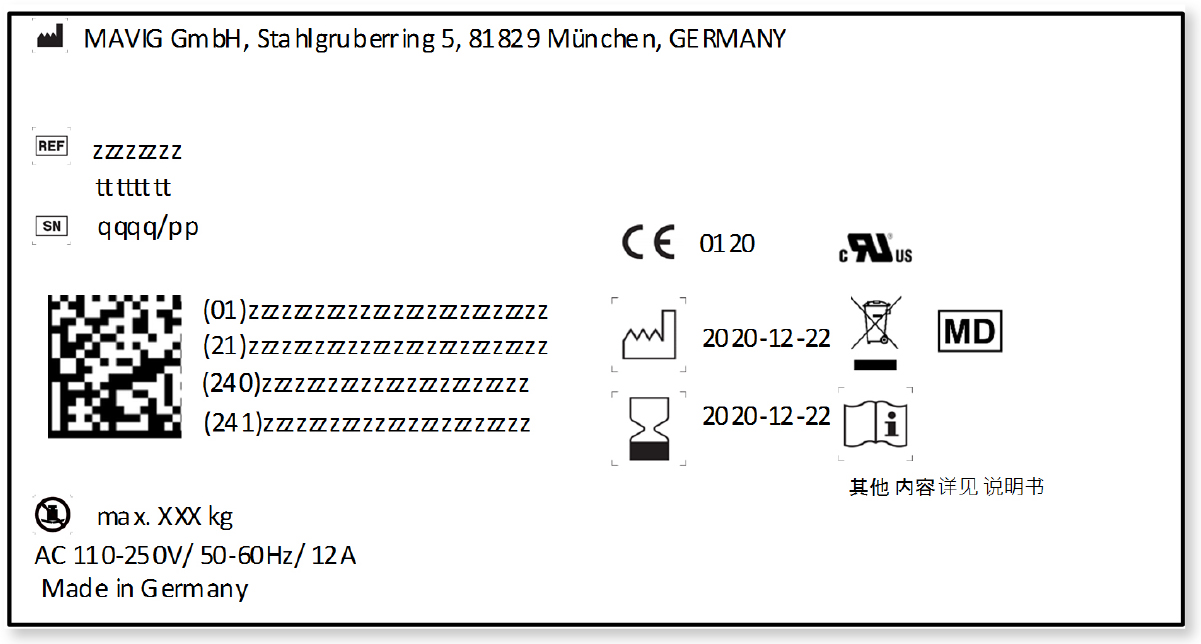
EU MDR – Medical Device Labeling Changes & Challenges - Regulatory, Clinical Consulting Services to Biopharma & Medical Device Companies