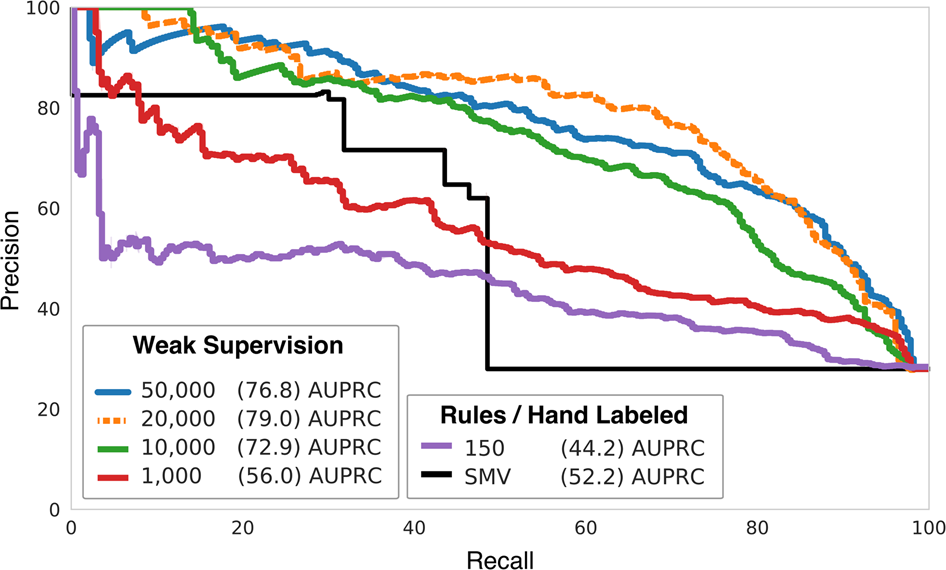
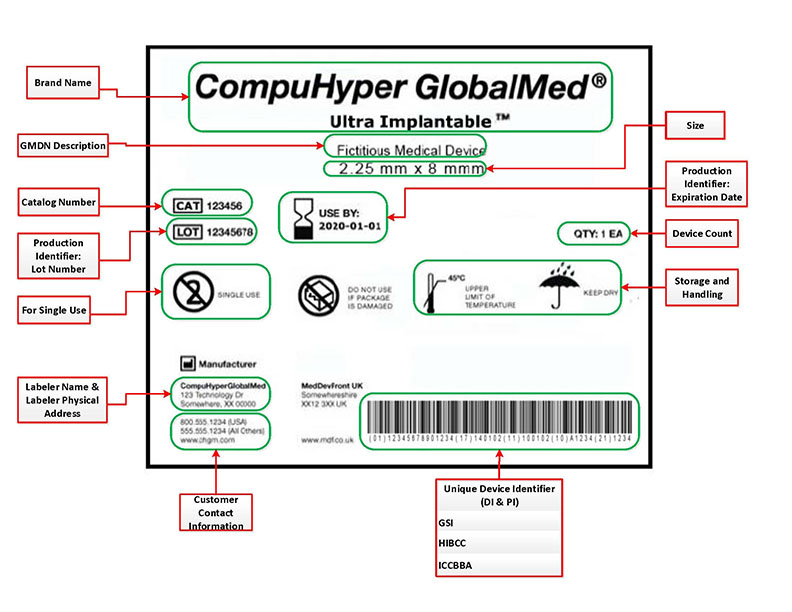
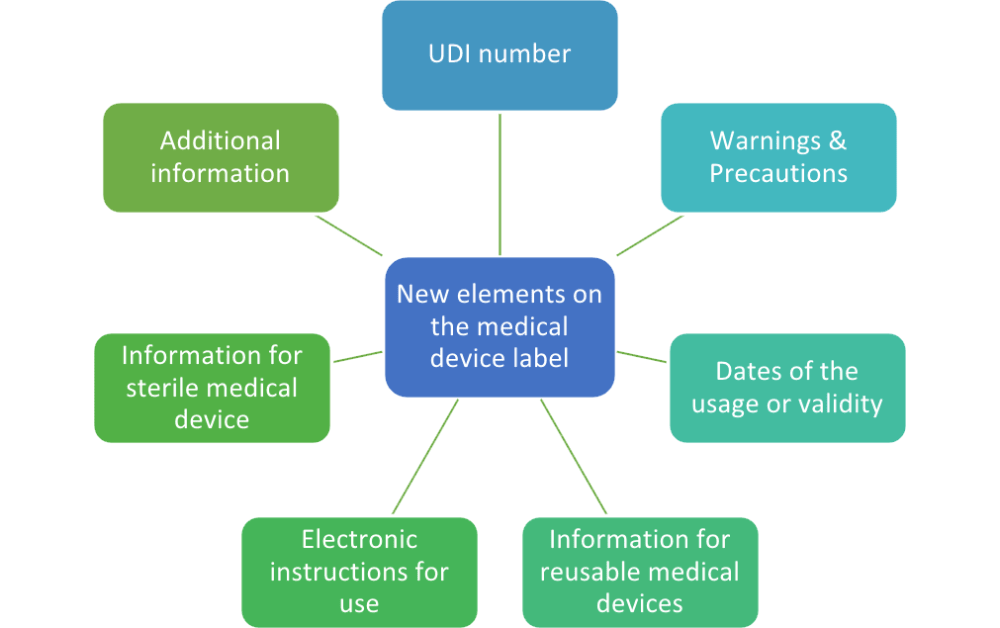
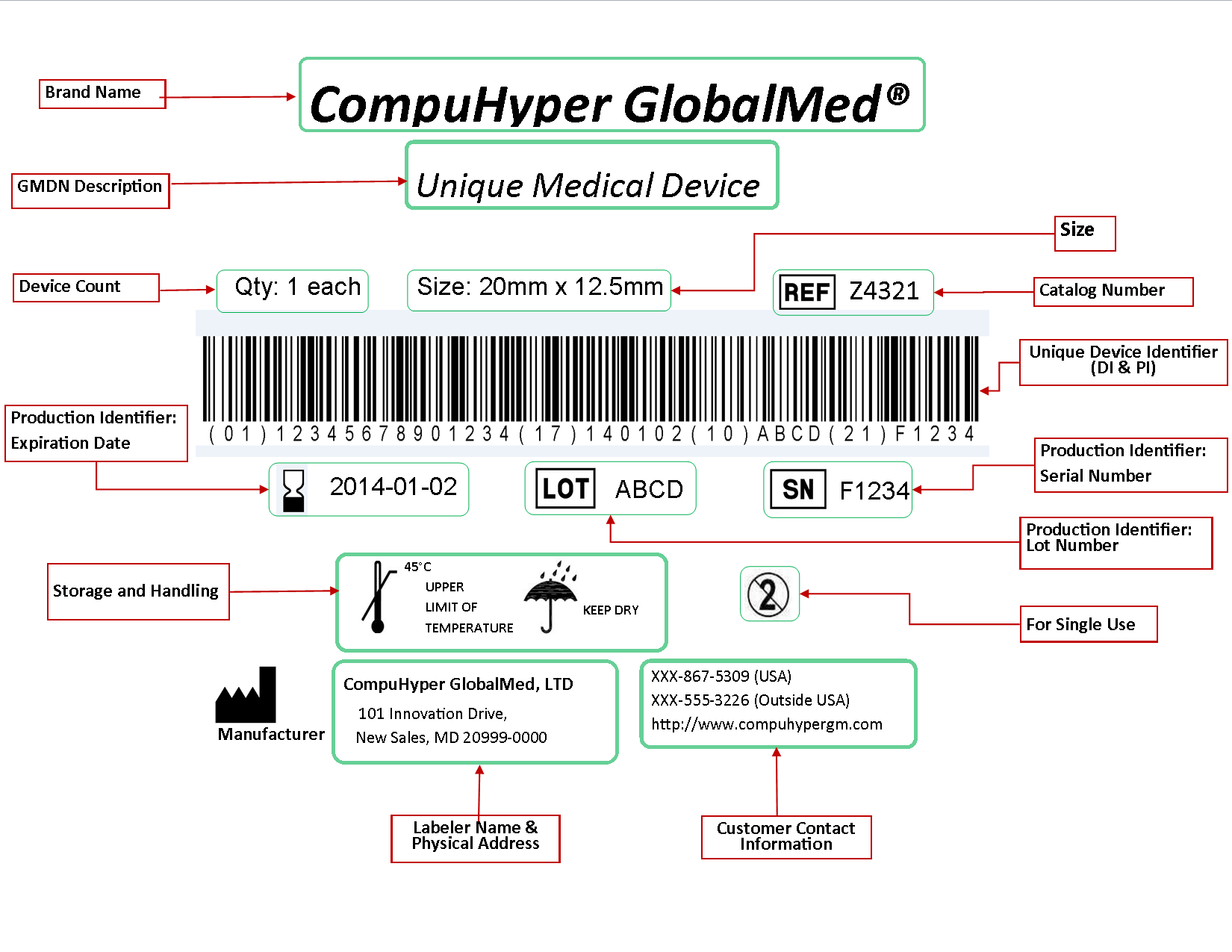
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR): What's the Difference?

Concepts for the Risk-Based Regulation of Clinical Research on Medicines and Medical Devices | Semantic Scholar
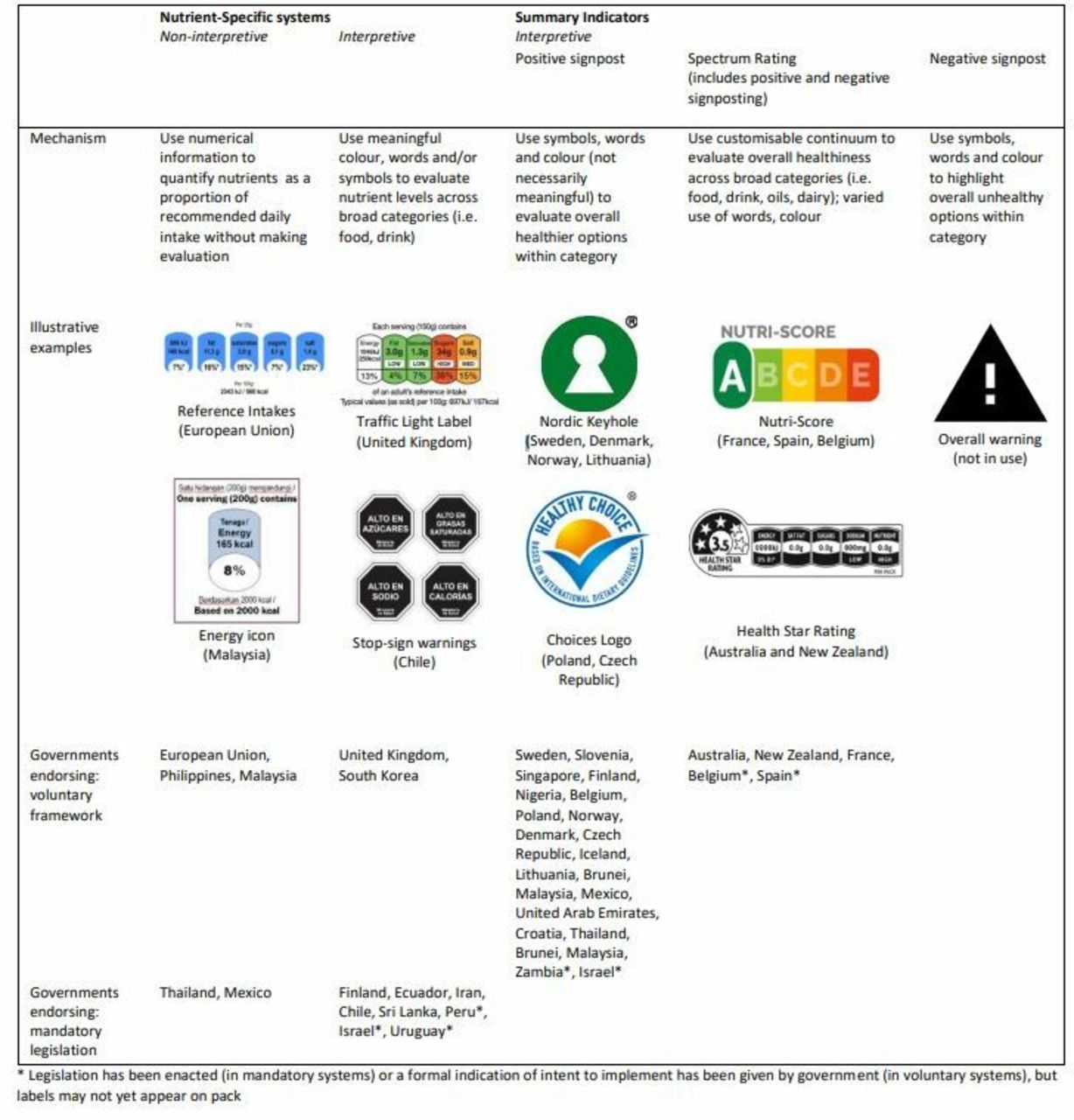
Front-of-pack nutrition labelling to promote healthier diets: current practice and opportunities to strengthen regulation worldwide | BMJ Global Health

Health Canada's Action Plan on Medical Devices: Continuously Improving Safety, Effectiveness and Quality - Canada.ca