KaftrioNOW: The Buzz Tag For Cystic Fibrosis That Is Trending On Social Media | by Emma Boniface | Coughy and Creon | Medium

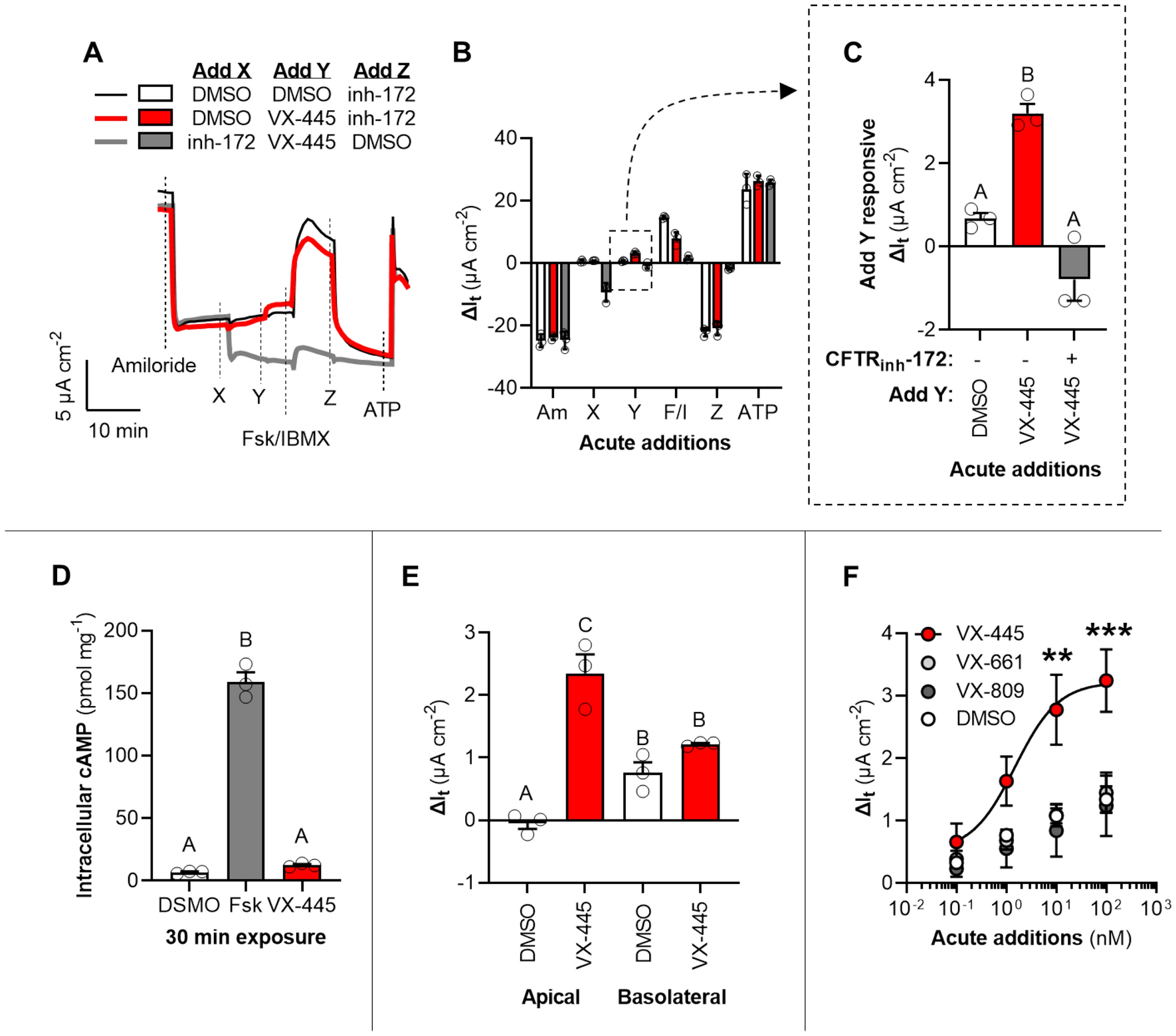
Long-term safety and efficacy of tezacaftor–ivacaftor in individuals with cystic fibrosis aged 12 years or older who are homozygous or heterozygous for Phe508del CFTR (EXTEND): an open-label extension study - The Lancet

Share petition · Ministère de la sante: Accord France Vertex MAINTENANT contre la mucoviscidose · Change.org

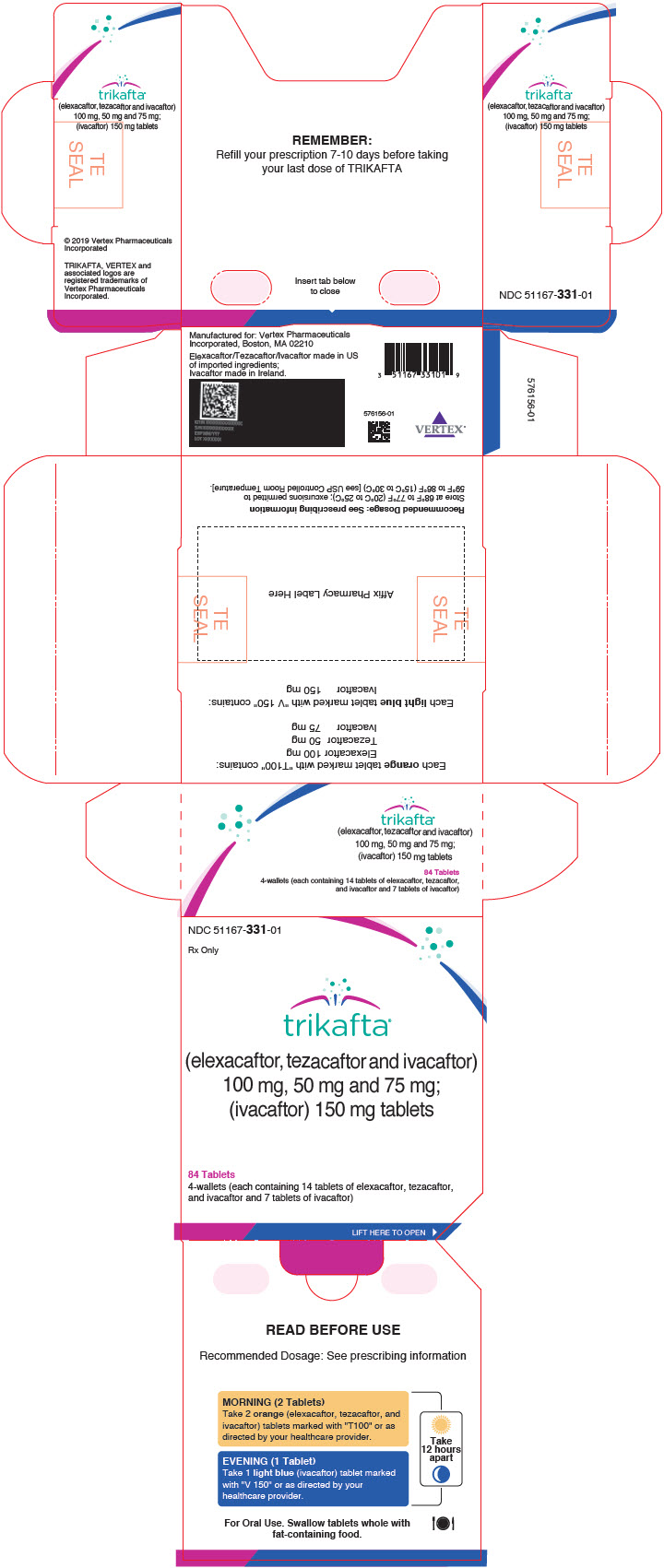
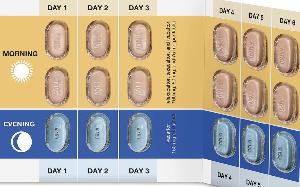
Vertex Announces FDA Approvals of TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor), SYMDEKO® (tezacaftor/ivacaftor and ivacaftor) and KALYDECO® (ivacaftor) for Use in People With CF With Certain Rare Mutations | Business Wire

Vertex's Trikafta gets FDA approval to treat cystic fibrosis in children - Pharmaceutical Technology